The Standard for High Acid Producing Soils Helps Protect People and Wildlife

The gray-green soil in the photo indicates high acid-producing soil with a pH of 4 or less. The soil was uncovered during construction of this developed site. The Standards for Soil Erosion and Sediment Control (P.L. 1975, chapter 251, N.J.S.A. 4:24-39 et seq) provides regulatory guidelines for managing potentially harmful high acid-producing soils. Burying the soil with alkaline limestone and covering it with a minimum of 12 inches of settled soil with a pH of 5 or higher will protect onsite soils and offsite streams and lakes from sulfuric acid leachate. Soils with a pH of 4 or less are unsuitable for growth of vegetation. Areas where trees and shrubs are to be planted shall be covered with a minimum of 24 inches of soil with a pH of 5 or higher.
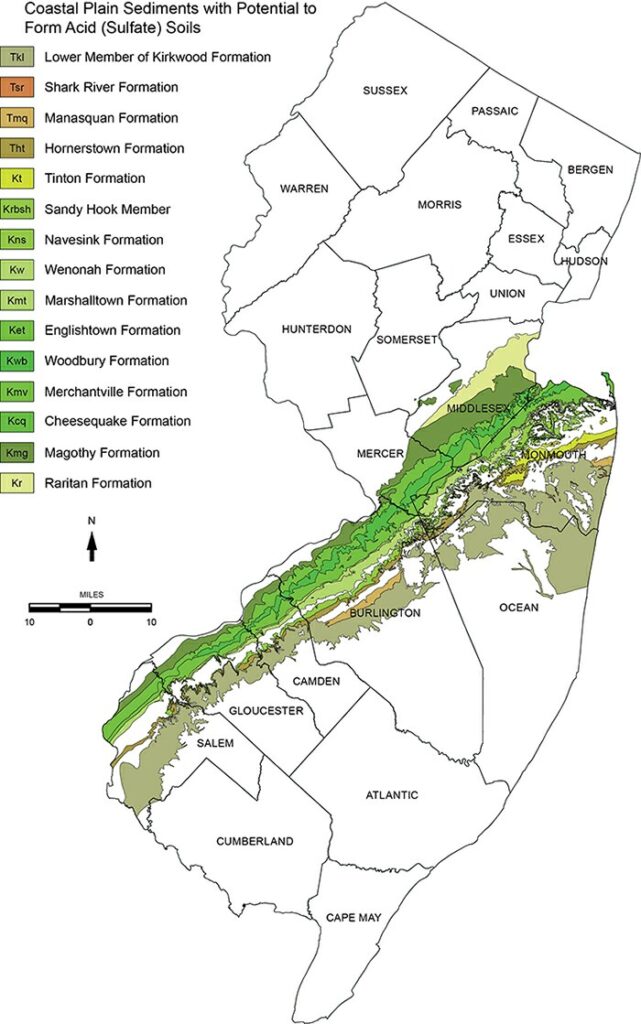
| A band of highly acidic soils are predominantly found within the inner coastal plain and parts of the outer coastal plain of south Jersey. Click to explore the NJDEP Bureau of GIS interactive map showing potential acid-producing (sulfate) sediments and their geologic formations. Soil pH is a measurement of the concentration of hydrogen ions (H+) found in soil water, and indicates acidity or alkalinity. Soil acidity can be the result of naturally acidic parent material, it can occur through rainfall and leaching, or it can develop through the process of decomposition of organic matter which produces hydrogen ions. Soil is complex! But it’s also truly amazing. For more information about the acid soil forming process visit the NJ Bureau of GIS website. |
